
Almost all industries use monitoring and measuring equipment to manufacture products or support services, it is required that this equipment must be calibrated to ensure that the intended product specification is achieved. In short, quality is maintained when calibration is performed.
ISO 9001 is the quality management system designed for manufacturers (producers of products) or service providers. There are other more specific standards for specialized industries like automotive, pharmaceuticals, or Oil and Gas but ISO 9001 is still integrated with them.
For a calibration process to be properly implemented, there should be in-house calibration management that we should follow.
What is calibration and why do we need calibration? Visit my other post in this link >> calibration awareness
This calibration management is guided by ISO 9001: 2015 Standards which have dedicated clauses that detail the requirements needed for proper implementation.
In this article, I will share with you:
1. The related clauses of ISO 9001 are focused mainly on calibration requirements.
2. ISO 9001 Calibration procedure – The content of a calibration procedure
Read on…
ISO 9001 Calibration Requirements
What are the Calibration requirements of ISO 9001? Below are the related clauses where calibration requirements are provided. The clauses that I listed here are the clauses with calibration requirements that are directly affecting the calibration results, these are:
1. Clause 7.1.2 People
2. Clause 7.1.4 Environment for the operation of processes
3. Clause 7.1.5.1 General monitoring and measuring requirements
4. Clause 7.1.5.2 Measurement traceability
5. Clause 7.2 Competence
6. Clause 9.1.1 General requirements for monitoring, measurement, analysis, and evaluation
These are the calibration requirements as per my experience, you may include other clauses you know that are directly affecting the calibration results.
Clause 7.1.4 Environment for the operation of processes
This clause requires the monitoring and control of the environment for the correct performance of calibration. Same with ISO 17025 requirements, environmental conditions that influence the final output of calibration results should be monitored and controlled.
Some examples of environmental conditions that we need to monitor and control are:
1. Temperature
2. Humidity
3. Vibrations
4. Dust
5. Proper lighting
6. Airflow
Not all listed above need to be controlled simultaneously inside a lab. This depends on the criticality and the effect it can have to the calibration performed.
But mostly, the two environmental conditions that are always controlled are temperature and humidity because almost all instruments require it for proper functioning, it is detailed in their specifications.
Clause 7.1.5. monitoring and measuring requirements
Instruments that we use to perform measurements are considered monitoring and measuring instruments.
“It is either we perform a measurement to monitor and control a process or we perform measurement for verification of the output of our process.”
With this in mind, all the instruments that are used for monitoring and measurement should be controlled.
The control provided are:
- The instrument to be used should be suitable. Suitable means it covers the range and accuracy requirement. For every monitoring and measuring instrument we use, we should ensure that the usable range can be covered and as much as possible, it has higher accuracy than the process to be measured. Recommended is to maintain the Test Uncertainty Ratio (TUR) of 4:1.
Maintaining a TUR of at least 4:1 will ensure that our standards are still in confidence even it encounter a drift that we cannot immediately detect.
Another best approach is to use a standard with small measurement uncertainty results, this can be seen in its calibration report. Smaller measurement uncertainty means more accurate output of our calibrators.
2. Every monitoring and measuring instrument should be maintained to ensure confidence while using or within its calibration interval. Maintained means:
a. Properly monitored for its statuses like locations, labels, and calibration due dates.
b. Preventive maintenance is performed
c. Intermediate check is performed – a verification process.
Records of Implementation of the above requirements should be maintained that can be used as evidence of implementation during audits.
Check out this link to read more about monitoring and measurement >> Applications of Monitoring and Measurements
Clause 7.1.5.2 Measurement traceability
We should ensure measurement traceability for every monitoring and measuring instrument used. It is a requirement that is a must to follow to ensure confidence in the validity of measurement results.
Let us define first Traceability. As per VIM 3 clause 2.41, Traceability or Metrological Traceability is the “Property of a measurement result whereby the result can be related to a reference through a documented unbroken chain of calibrations, each contributing to the measurement uncertainty”.
There are 2 ways where we can implement Measurement Traceability requirements, these are:
1. Establishing traceability
2. Maintaining traceability
Establishing Traceability
In establishing traceability under ISO 9001, we use clause 7.1.5.2 stating that,
The definition above is about establishing Traceability, wherein the measurement result of our instruments should be linked or connected through an unbroken chain of calibrations all the way to SI.
(a) “all monitoring and measuring instruments shall be calibrated or verified, or both, at specified intervals, or prior to use, against measurement standards traceable to international or national measurement standards”.
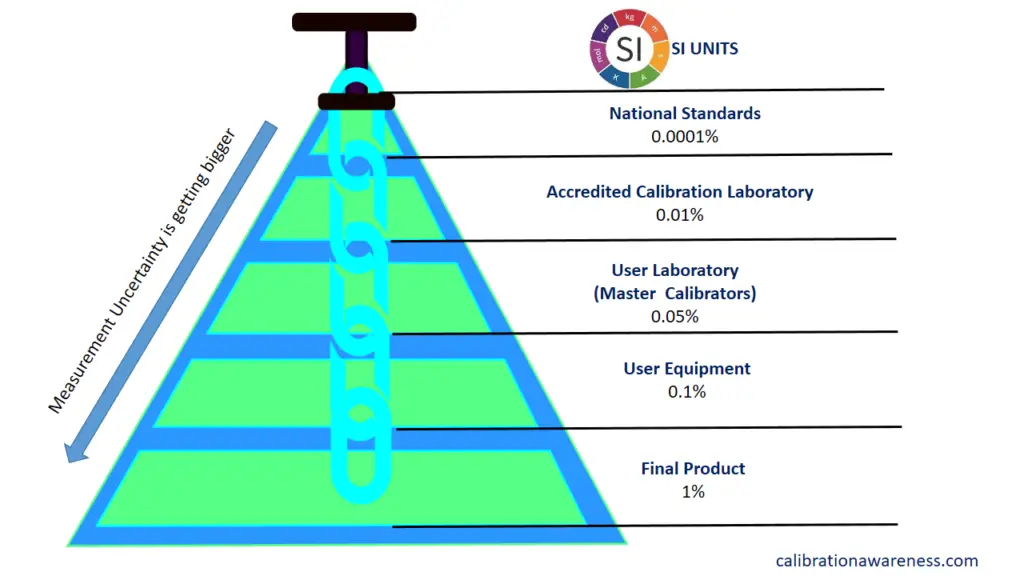
The traceability of your instruments will be established when calibrated, verified or both prior to use, by a higher laboratory that is linked to international standards or SI.
How is traceability established during calibration? One objective answer to this is through the “measurement uncertainty” results. After calibration from a higher lab, a link is connected once there is a Measurement Uncertainty results reported in the calibration certificate. This is the value in the traceability pyramid that is being passed on from the top to the bottom. Read more at this link >> The Deeper Meaning of Traceability in Calibration
To ensure traceability, such laboratories should be accredited under ISO 17025. Traceability can also be established by using a Certified Reference Material or CRM provided by a reputable source.
Maintaining Traceability
After the successful establishment of traceability, the most challenging part is now to maintain traceability.
Activities that support the maintenance of traceability are the following which are the 2 remaining sub-clauses of 7.1.5.2. these are:
(b) Identified in order to determine their status
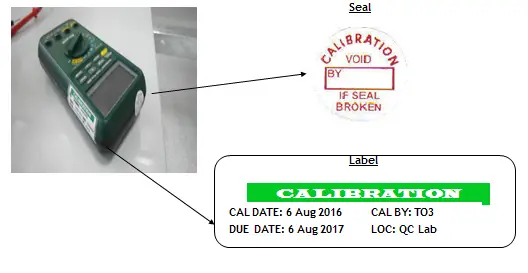
Identification through labeling and coding of measuring instruments in order to monitor their status. Labels include a clear detail of its calibration due date, date of calibration, and who performs the calibration.
Status means their present location, calibration interval, calibration condition (within tolerance), and specific records that detail their performance and specifications.
(c) safeguarded from adjustments, damage, or deterioration that would invalidate the calibration status and subsequent measurement results.
Safeguarded from adjustments using void seals and safeguarded from damage or deterioration by implementing procedures for proper handling, transport, and storage while it is being used for measurement and/or calibration.

When measuring equipment is found to be unfit for its intended purpose, appropriate actions shall be taken like:
1. recalibration,
2. adjustment and verification
3. adjustment of the calibration interval
4. proper disposition – scrap or reject
Read more about the deeper meaning of traceability in this link >> Traceability
Clause 7.2 Competence
As defined by ISO 19011 competence is: “demonstrated personal attributes and demonstrated ability to apply knowledge and skills “.
Clause 7.2 and 7.1.2, which is about People are related to each other. ISO 9001 clause 7.1.2 requires that “The organization shall determine and provide the persons necessary for the effective implementation of its quality management system and for the operation and control of its processes.”
In relation to calibration, the People, which are the personnel involved in performing calibration should be competent or have the necessary competency.
Personnel Competency is one of the main requirements that we must meet. All factors that influence the quality of calibration performed depend on the knowledge, skills, experience, and education of Personnel.
You may have a high-end calibrator, a good calibration procedure, and a well-equipped facility but the person in charge is not suitable for the calibration activity performed, then the calibration results may be invalidated.
To be competent means:
1. Has the necessary competence requirements such as appropriate education, training, or experience;
2. Pass the competency evaluation
3. Authorized to perform important laboratory activities
4. Monitored competency and continued education
“Top-notch calibrator, proper procedure, and excellent facility won’t guarantee valid calibration results if the person in charge isn’t suitable for the task. People matter in calibration!”
All the records and recorded information resulting from this process should be maintained and used as evidence of competency.
I discussed more ‘personnel competency’ in my other post here >> Personnel Competency
Clause 9.1.1 General requirements for monitoring, measurement, analysis, and evaluation
This clause is composed of many processes or activities that involve monitoring, measurement analysis, and evaluation. But in this article, we will just focus on the requirements related to calibration.
In these requirements, we need to determine:
1. What needs to be monitored and measured.
We need to monitor our measuring instruments and measure or perform verification on the measurement results or displayed output.
We need to monitor measuring resources to be able to control and properly maintained and ensure the validity of results every time they are used. Monitoring is easily implemented by having a master list of all the measuring instruments and standards that details their important information and specifications including their calibration records.
2. The methods for monitoring, measurement, analysis, and evaluation needed to ensure valid results;
We need to have a procedure for quality control like performing recalibration, intermediate check, calibration interval adjustment, or verification and validation to ensure traceability and valid results are maintained. We can use statistical techniques like control charts or a simple checklist and tables to analyze and evaluate each performance.
3. When the monitoring and measuring shall be performed;
All the methods or procedures for quality control stated in number 2 (above) should have a specific schedule of implementation. For example, for Temperature and humidity, a schedule of 3 times a day is given. Another example is the Intermediate check which is performed every 3 months alongside preventive maintenance. This will ensure regular or periodic implementation to maintain the quality and confidence of results.
4. When the results from monitoring and measurement shall be analyzed and evaluated.
Every time we implement the quality control procedures of number 3 above, we can gather the data then immediately subject to analysis and evaluation.
Another example of analyzing and evaluating measurement results is during the receipt of a newly issued calibration certificate. There should be a process in place on how to review or evaluate a calibration report.
Mostly, we are looking for any out-of-specification condition or a trend that shows us something is wrong or will become an issue in the future. If any issues were found, corrective or preventive action will be issued and proper action is taken.
Other Calibration Requirements
The clauses that I identified above are the main clauses that have an impact on the calibration results and are directly calibration implementation requirements in my understanding. There are other clauses that are still calibration-related but do not directly impact the results of calibration and are mostly under Quality.
Such as:
1. Internal audit – auditing the calibration process
2. Document and records control – controlling documents and records like calibration procedures, calibration certificates and datasheets.
3. Risk and opportunities – Identifying risk and opportunities from calibration activities to improve and mitigate any issues found.
4. Improvements – performing improvement activities to further improve the calibration processes like equipment upgrades, regular training, or monthly meetings.
5. Complaints or Customer survey – customers are the users of instruments, it is important to document their concerns for improvements.
Overall, the requirements of ISO 9001 will provide a complete implementation to support the proper operation of an Internal Calibration Laboratory. But we need to have a deeper understanding of all the calibration requirements in order to properly manage a calibration Lab.
ISO 9001 standards do not provide the exact Instruction to manage a calibration lab, just a general requirement. If you want in-depth Calibration Laboratory management guidelines, you need to be familiarized with ISO 17025 Standards, this is the main standards for calibration and testing lab, specifically if you want to provide services to other clients or industries.
Do you want a guide in implementing In-house Calibration? Check out my other post here>> Elements In Implementing an Internal Calibration Laboratory
ISO 9001 Calibration Procedure
I will share here the ISO 9001 calibration procedure, also known as the calibration method, which is referring to an Internal calibration procedure used to execute the actual calibration of instruments for in-house or internal calibration lab.
I have seen a number of calibration procedures from different calibration labs and I can tell you that every company has different formats.
There are no exact requirements for the formats of a calibration procedure that I am aware of, most available formats are just recommendations. I believe it is based on the company policy and the experience or expertise of the Calibration engineer who created the calibration procedure.
There are some instances that a calibration procedure for ISO 9001 is very simple, where it is integrated into the procedures of preventive maintenance or equipment operation manual.
There are also calibration procedures that are ‘generic’, which means it is applicable for many types of instruments.
But regardless of either ISO 9001 or ISO 17025 calibration procedure, before we will use it, we must consider the important calibration procedure requirements, these should be:
1. Verified or validated before use.
2. It has traceability; there is a reference to where it came from and who created it.
3. Approved before use
4. Up to date – the latest version is used
5. Controlled- included in Document and Records Control
Content of a Calibration Procedure
If you are planning to create or prepare an ISO 9001 calibration procedure or calibration methods, the following are the calibration procedure content, as a minimum, that I am aware of.
These can be used as content of a calibration procedure template.
These are:
1. Purpose – state the reason why we have the calibration procedure
2. Scope–to what instruments does the procedure applies including the range if necessary. (sometimes purpose and scope are the same).
3. Terminology – includes here the terms or acronyms used in the procedure that we need to define.
4. Safety–detailed here the possible safety hazards and their avoidance including any safety documents that need to follow or accomplish before performing calibration.
5. Reference Standard Used–describes all the reference equipment used for calibration for traceability and accuracy evaluation.
6. Other Materials/Tools – includes all other supporting materials or tools to perform the calibration/test activity.
7. Reference Document Use–these are the reference documents where the procedure was based during write-up or drafting. Also, a reference to determine the traceability of the procedure used.
8. Initial requirements/preparation– as the term implies, any requirements or prerequisites before initiating the calibration/test process. A preparation in order to achieve the best results.
9. Calibration Procedure -Main Steps of procedure Execution, the step-by-step instructions in performing the actual calibration. This includes any calculations or photos to easily describe and understand the procedure.
10. Acceptance Criteria–detailed here is the tolerance limit that needs to be met for verification purposes after performing the calibration.
11. Storage and Handling – describe here where to put the instruments and how it is handled after calibration.
Additional that you can include are:
1. Responsibility
2. Summary of Procedure
3. Not operational conditions
4. Accuracy and /or Components of Measurement Uncertainty
5. Calibration Status
6. Calibration Certificate Content
7. Correction or Adjustment procedure
8. General Notes
ISO 22000 Calibration Requirements
Calibration is very important not just in the manufacturing sector but also in the Food Industry. It is one of the main requirements in order to properly manage Food Safety.
Calibration is needed for reliable and accurate monitoring and measuring instruments. With the importance of calibration in the food industry specifically in maintaining food safety, the following are the requirements of ISO 22000:2018 to control monitoring and measuring instruments:
- calibrated or verified at specified intervals prior to use;
- when necessary, an adjustment should be performed;
- calibration status should be identified;
- safeguarded from adjustments that would invalidate the measurement or calibration results;
- properly handled to protect from damage and deterioration;
- calibration and verification results are documented and retained;
- calibration performed is traceable to international standards;
- measurement results are reviewed and verified to conform with the requirements and specifications, any non-conformances are dealt with appropriate actions.
I know that this article is focused on ISO 9001 calibration requirements, but upon checking it with ISO 22000, Food Safety Management System, there are almost no differences between ISO 9001 and ISO 22000 in terms of calibration requirements. Therefore, you can apply everything that I have discussed above.
Conclusion
ISO 9001 Standards is a Quality Management System that is well known and applicable for almost all industries. One of the major requirements under this standard is the implementation of calibration.
Calibration provides quality, reliability, and safety of manufactured products, therefore, it has a dedicated clause focused mainly on implementing the calibration process.
In this post, I have shared the following:
1. The related clause of ISO 9001 are focused mainly on calibration requirements. These are:
-
- Clause 7.1.2 People.
- Clause 7.1.4 Environment for the operation of processes.
- Clause 7.1.5.1 General monitoring and measuring requirements
- Clause 7.1.5.2 Measurement traceability
- Clause 7.2 Competence
- Clause 9.1.1 General requirements for monitoring, measurement, analysis, and evaluation.
2. ISO 9001 Calibration procedure – The content of a calibration procedure
If you want an in-depth understanding of calibration management and calibration processes, you can use ISO 17025:2017 as the Quality Management System. This Standard is dedicated to testing and calibration laboratories.
Thanks for reading, if you want to say Thank You, you can buy me a coffee.
Edwin
24 Responses
M. saleh AL-Mufti
Dear Eng. Edwin,
I’m very grateful for your efforts & kind attention to calibration issues.
Q., How do I know that the calibration certificate that I issued meets the requirements of specification ISO/IEC 17025: 2017 accurately?
Thanks in advance.
edsponce
Dear Sir,
You are welcome, I appreciate the time for reading my posts.
To answer your concern:
There are 2 ways to check the content of the calibration certificate, 1st is to check the requirements of a calibration certificate and 2nd is to check the requirements of the customer- the calibration results.
1. To determine if you covered all the requirements of a calibration certificate, you can use ISO 17025 clause 7.8.2 as a checklist. All the requirements for a calibration report are listed there.
2. Regarding the calibration results, it is based on the calibration lab procedure and the agreement during contract review or the assessment of customer requirements. This should be documented for example, on the Quotation or purchase order (PO), sometimes, there is a customer request form.
The key here, in order to meet the specifications accurately, is to have a quality person that is familiar with the standards (ISO 17025). He/She is the authorized person to review and check all the requirements prior to issuing the calibration report.
You can read more about calibration certificates in my other posts here >> How to Properly Use and Interpret an ISO 17025 Calibration Certificate
Thanks and regards,
Edwin
M. Saleh
Thanks for your reply.
edsponce
Hi Sir,
Happy to help.
Edwin
Chris
Hello. Would you be able to point me to any ISO or other standard which lays out geophone calibration requirements (e.g., timing thereof relative to work) on construction sites? Thank you.
edsponce
Hi Chris,
Sorry, but I am not yet aware of such procedure or standard if there is. I will update you just in case.
Thanks for visiting my site.
Edwin
Adriana Belkova
Dear Mr. Edwin,
I have a question regarding in-house calibration performed with working/factory reference standard traceable to national standard:
Is calculation of measurement uncertainty of in-house calibration required? Or is it sufficient if calibration result is reported as deviation from working/factory reference standard, without associated measurement uncertainty?
Thank you for your reply in advance.
Adriana
edsponce
Hi Adriana,
It is not required for in-house calibration if you are not following a Standard’s requirement like the ISO 17025.
But, since measurement uncertainty is one of the basis to show how accurate your measurement is, there must be a way where you must demonstrate that the standard you use or the calibration you performed is not affected by any inaccuracy or errors that may be present.
Deviation and measurement uncertainty is not the same therefore, it cannot replace the measurement uncertainty.
One way to solve this is by having a procedure where you only accept or perform calibration work without calculating measurement uncertainty if you ensure a TUR (Test Uncertainty Ratio) of above 4:1.
TUR = tolerance limit of the DUT/expanded measurement uncertainty of standard.
You can check more about TUR in this link >> https://calibrationawareness.com/8-ways-how-you-can-use-the-measurement-uncertainty-in-a-calibration-certificate
I hope this helps,
Edwin
shivam ramani
Hello Sir you gave a very detailed explanation. Thank you for such a wonderful explanation.
Question : we have some templates which we use to verify the position of critical characteristics of items. Now, it does not directly affect the quality. Can we exclude those from our internal lab scope and say that we do not control those internally.? or it can be a minor since it is being used in process and has to have some kind of control.
Also, what are specific requirements for scope of lab.? as in should it have all the master calibrator specs like range, resolution etc or we just need to cover what equipmeenst we will calibrate internally.?
edsponce
Hi Shivam,
You’re welcome, I appreciate the time to read my post.
If it does not directly affect the quality of your products or processes, then yes, you can exclude it, but as the expert in that parameter, you should have proper documentation on why you have excluded it. But if you think it has a significant purpose or use in the process even though it is not critical, it is good to have some kind of control, you may provide a longer calibration interval as an example.
I have created a good guide if you want to “tag” it as ”calibration-not-required”. Check out this link >> https://calibrationawareness.com/calibration-not-required-implementation-guide-for-in-house-calibration
Regarding the lab scope, there are no specific requirements for a scope of lab if you are managing an internal calibration lab, but as per my experience on other lab scopes that are required by accreditation bodies, then Yes, you need to include the basic specs like make/model/range/serial number/uncertainty/accuracy, then alongside with it are the UUTs that you can calibrate.
I hope this helps,
Best regards,
Edwin
Sarah
Wonderful info..! That’s a great post that described and provides in-depth knowledge of the ISO 9001 Calibration Requirements -Calibration Clauses. The ISO 9001 standard is important for every organization who connected to any industry and want to achieve the trust of its customer. Visit here: https://www.punyamacademy.com/course/quality/iso-9001-2015-requirements
edsponce
Thank you Sarah for reading my post!
Dipesh Shrestha
Dear Edwin sir,
Your blog is a great compilation of knowledge base for calibration and Quality professionals. Pls keep it up. We love it.
edsponce
Hi Dipesh,
I am glad you love it. Appreciate the comments.
Have a safe day,
Edwin
Tammi
Edwin,
Does ISO recognize a “In-Service” date as a valid reason for a gauge to be outside of its calibration due date? Or, is the due date the due date and it is clearly expired?
edsponce
Hi Tammi,
ISO 9001 or 17025 does not recognize the “in-service” date as a valid reason to determine if a gauge is due or not. What is recognized is the calibration date and due date written on its label or calibration sticker that is supported by a calibration certificate.
However, the In-service date can be used as verification evidence to extend or lessen the calibration interval of the gauge.
I hope this helps,
Edwin
Akash Kumar Prajapati
Hi Edwin,
Such an informative post. I have a question related to calibration schedule compliance. In any industry if any pressure gauge or weighing scale is being used in regular operation. What should be the calibration frequency and what will be the acceptance criteria for delay in calibration. For example if calibration of a weighing scale is due on 15th March, should it be calibrated by 15th only / before 15th or it can be calibrated after 15th march. If after it can be calibrated then what is the accepted no. of delays means same month / 15 days / 30 days or something else.
edsponce
Hi Akash,
I want you to know that there is no fixed rules regarding calibration interval. It is always dependent on the user. The challenge here is where should you base your initial calibration interval.
Calibration frequency or interval has mostly defaulted to 1-year interval. The majority is based on manufacturer recommendations or as per experience with the same type of instruments.
But as time goes by where you have gathered a good history of data, this is now the time when you can establish a suitable calibration interval for your instruments.
If the calibration of your weighing scale is due on March 15, then it should be calibrated either earlier date or on the exact date, else it will become overdue in which the scale is already unacceptable to be used. Unless you made a policy or procedure that calibration is only due Plus 15 days more for example. But remember this should be documented in your procedure. The number of days you specify is based on your study that the scale is still in tolerance within these additional days.
You can read more about calibration interval on my other post in this link >> calibration interval
Thanks for reading my posts.
Edwin
Alan
We have various steel measuring tapes that had previously had calibration labels on them, these have lapsed because the Factory Manager performed the task has left. We would prefer an in house option to re-calibrate, would a starting point be establishing competence of a new calibrator (through training) to satisfy 9001?
edsponce
Hi Alan,
Yes, that would be a good option since I believe you still have the calibrator or standard equipment to calibrate them.
What you need in order to satisfy ISO 9001 are:
1. A traceable reference standard, preferably calibrated by an ISO 17025 accredited laboratory.
2. A competent personnel- with training and qualifications for the job.
3. An approved calibration procedure
4. Proper documentation.
The above are the main requirements, for more details, you may visit my other related article in this link >> In-house calibration
Thanks and regards,
Edwin
Yesenia Hernandez
I have several Analytical Balances that need to be calibrated for an ISO 9001 audit coming up. We are NOT FDA regulated since we are only producing products strictly for RESEARCH purposes only. And the Analytical Balances have an internal Calibration System embedded in them, my question is:
If I calibrate the Analytical Balances using only the Internal built-in calibration system (by following the internal calibration instructions found on the balance manual) is it SUFFICIENT for ISO 9001 requirements? or do I still need to use accredited calibrated weights to calibrate externally?
edsponce
Hi Yesenia,
Internal calibration or the auto-calibration embedded in the balance is not acceptable as calibration evidence for ISO 9001 requirements unless the internal weights embedded are also calibrated, which means it has a valid calibration certificate with it.
The reason why it is not acceptable are:
1. There is no traceability or it is not linked to a higher standard going to an International Standard or SI.
2. Accuracy of embedded calibration weights is not guaranteed because it has no calibration certificate to show this.
3. Proper calibration procedure is not performed. A validated or accepted calibration procedure should be available.
What you can do is to use calibrated weights, calibrated by an accredited laboratory after performing the internal calibration of the balance. You should have an acceptable procedure for this and record it in a calibration report or certificate.
Another is to have it calibrated by an accredited laboratory. This is the best thing to do if you do not have your own standard weights.
I hope this helps,
Edwin
Raymond Sims
Good morning. This is great information. I used to be one of the calibration techs that just “filed away” the documentation without a full review. I learned that lesson the hard way. I have a quick question for you, for the signatures, can the person performing the calibration be the same as the person approving the certificate? I know there has to be a technical and quality review of the certificate. The reason I ask is ISO 17025 requires the identity of the person authorizing the reports. ANSI N323 (for radiation instrumentation) requires the identity of the person performing the calibration. NIST’s SOP states that calibration certificates: “Clearly identify the person(s) performing the calibration and authorizing calibration certificate by stating their corresponding name, title, and signature. Each authorized signatory accepts responsibility for the technical accuracy and validity of the reported results.”. It appears that they can be one and the same. Any clarification on this matter will be greatly appreciated.
edsponce
Hi Raymond,
Good day!
My answer to your question is, yes, the person performing the calibration can also approve the certificate as long as he/she is the authorized signatory. But as much as possible, we avoid this single-person approval because of the requirement of Impartiality. Approving your own work poses a threat to impartiality or there is a risk to impartiality. We can avoid this risk by having a 2nd person reviewing and authorizing the report, this can be the function of the quality.
Calibration certificate approval should be a shared responsibility, one focusing on the correctness of the calibration data (as per the calibration procedure used) and one focusing on the completeness of the totality of the certificate (as per the calibration certificate requirements-ISO 17025).
I hope this helps, thanks for reading my post.
Edwin