Calibration is one of the most important aspects when we are engaged directly or indirectly in manufacturing a certain product in terms of measurements.
Almost all of the industries require this service mainly because of quality and safety (and of course including auditor’s requirement).
Because of this, the Internal calibration laboratory is established and many 3rd party calibration laboratories exist to provide their services.
Calibration service allocates a significant amount of a company’s budget and therefore it is a big plus if you consider having an internal calibration laboratory also called in-house calibration to be implemented.
In this topic, I will share my knowledge based on my experience regarding the elements or guides when establishing an Internal Calibration Laboratory which is aligned to ISO calibration requirements specifically ISO9001:2015 and ISO17025:2017. You will discover the requirements in order to implement a calibration system within your company including below topics:
-
- The Benefits of implementing an in-house calibration laboratory;
- Some challenges that you will encounter during implementation;
- Understanding the clauses and implementing the requirements of ISO 9001:2015 related to calibration
- The 13 elements that you need to have when implementing an in-house calibration laboratory.
I also presented here a basic Calibration process flow. This is not a guaranteed to be complete but, this is a good starting point to understand and implement your internal laboratory calibration system under ISO calibration requirements.
What are the Benefits of Implementing an In-house Calibration Lab?
What is In-house calibration? Also known as Internal calibration. Basically, it is a calibration system performed or established inside a company’s facility. This is to differentiate it from an external laboratory which is a third-party laboratory.
A third-party laboratory also has an in-house calibration program, where most of the processes discussed here are also applicable to it. But for simplicity, we will focus the discussion on the company-established calibration program or the in-house calibration.
The in-house calibration lab executes its own calibration system based on verified and accepted calibration procedures using traceable reference standards. This means that it is also aligned with the requirements of ISO 17025:2017.
If you have a lot of measuring equipment for calibration, you will save a significant amount of money (in terms of calibration from an outside source and logistics in the long run).
To verify whether you made the right choice in purchasing a standard, do a justification to compare how much you will spend with a single standard in comparison with how much you will pay for a calibration service with the related measurement equipment. See this link for a sample justification.
Here is a list of benefits of establishing an In-house calibration lab:
- You will save time in calibration downtime because of removing the need in sending your instruments to an outside lab.
- Customers have more trust in your performance in terms of monitoring the measurement process.
- More capabilities in terms of measurement will be explored.
- Calibration support is readily available when needs arise
- The possibility of providing calibration support to other locations or departments.
- Centralize execution of calibration and better monitoring. I observed that some companies have their equipment monitored as per department or by the person who utilizes the instruments; It is also their choice of whom they choose to provide calibration service.
- Non-Conforming calibration can be detected immediately, therefore, it can be isolated or the process affected can be informed immediately for proper assessment and disposition of the affected product.
- An additional safety feature is observed because the transferring part is reduced or eliminated
- Reduces the risk of vibrations or other environmental stress that it absorbs during transport thus, increased assurance of stability.
- You are implementing most, if not all the calibration requirements of ISO 9001:2015 Standards. See more explanation below.
Some Challenges in Implementing In-house Calibration:
- You need to manage a Quality System with wider calibration laboratory processes which include the generation and maintenance of calibration procedures, calibration certificates, preparation for a customer audit, and audit support to the various area of the industry.
- A master standard or reference standards are required to be purchased which also needs maintenance and calibration on a given calibration interval.
- Competent calibration personnel is needed which includes continuous training.
- You need to maintain the needed laboratory facility in order to support the achievement of valid results during calibration. This includes control of environmental conditions. (see element no. 9 below)
Implementation has some challenges, but once the calibration laboratory is established, implementation will become manageable.
Read on to learn and understand the ISO calibration requirements.
Understanding and Implementing ISO 9001:2015 Calibration Requirements
One of the main standards that require the implementation and proper execution of calibration is ISO 9001:2015. ISO 9001 calibration requirements do not differ from ISO 17025. Your knowledge of ISO 17025 is a big advantage when implementing ISO 9001 calibration requirements.
What are the Clauses in ISO9001 where the need for calibration is required, supported, and emphasized?
Below are the related clauses for calibration implementation under ISO 9001:2015: Clause 7.1.5 – Monitoring and Measuring Resources and Clause 9.1 – Monitoring, measurement, analysis, and Evaluation:
- Clause 7.1.4 Environment for the Operation of Processes
- The organization shall determine, provide and maintain the environment necessary for the operation of its processes and to achieve conformity of products and services. – managing an internal calibration lab or simply calibration implementation is one of the processes that contribute to product quality and therefore environmental control is necessary (see element no 10 below).
- Clause 7.1.5.1 General Monitoring and Measuring Requirements
- The organization shall determine and provide the resources needed to ensure valid and reliable results when monitoring or measuring is used to verify the conformity of products and services to requirements. (covers the 13 elements below).
- the resources provided are suitable for the specific type of monitoring and measurement activities being undertaken. (see element no. 1 below)
- the resources provided are maintained to ensure their continuing fitness for their purpose. (see element no. 13 below)
- The organization shall retain appropriate documented information as evidence of fitness for purpose of the monitoring and measurement resources. (see element 12 below)
- The organization shall determine and provide the resources needed to ensure valid and reliable results when monitoring or measuring is used to verify the conformity of products and services to requirements. (covers the 13 elements below).
- Clause 7.1.5.2 Measurement traceability
- In order to maintain proper traceability (unbroken chain of comparison) to provide confidence to measurement results, measuring equipment shall be calibrated or verified, or both, at specified intervals, or prior to use, against measurement standards traceable to international or national measurement standards (see element 9 below);
- properly identified in order to determine their status (see element 7 below);
- safeguarded from adjustments, damage, or deterioration that would invalidate the calibration status and subsequent measurement results. (see elements 5 and 7 below)
- The organization shall determine if the validity of previous measurement results has been adversely affected when measuring equipment is found to be unfit for its intended purpose, and shall take appropriate action as necessary. – this will fall under the process of “reviewing measurement results” and “handling out-of-tolerance conditions” which differs to every industry.
The important thing is that we have a system in place to backtrack and review necessary calibration data when problems are suspected in our calibration results in order to take the necessary actions.
Read more about this clause in this post. >>5 Mistakes When Using a Calibration Certificate that You Need to Correct
- Clause 9.1.1 General requirements for monitoring, measurement, analysis, and evaluation
- The organization shall determine:
a) what needs to be monitored and measured; (see element 1 below)b) the methods for monitoring, measurement, analysis and evaluation needed to ensure valid results; (see element 6 below)
c) when the monitoring and measuring shall be performed; (see element 1 below)
d) when the results from monitoring and measurement shall be analyzed and evaluated. (see element 13 below)
- The organization shall determine:
13 Elements for Implementing the Calibration Program in an Internal Laboratory.
1. Calibration Scope
Before we start our calibration project and gather the needed resources, it is important to consider first what is our calibration scope. This is where we can determine our capability of how wide our services can reach and what instruments we can calibrate.
This is the reason why we exist as an internal calibration lab. We will mention here the parameters that we can calibrate or the calibration service that we can provide, the reference standards that we are using with their accuracy or uncertainty value, and the reference procedure or methods including reference guides.
When can we perform monitoring and measurement?
As an in-house calibration lab, we should also determine when can we execute our calibration job for us to control calibration activities. Below are some reasons why or when can we perform calibration:
-
- Calibration is performed when a new instrument is installed or purchased
- instruments that are mishandled during transfer (for example: dropped or fell down)
- when instrument performance is questionable
- calibration period is overdue
- kept in an unstable environment for too long (exposed to vibrations or too high/low temperatures)
- when a new setting or adjustment is encountered
- when required by a 3rd party audit
- when required by customers
- when health and safety require monitoring
- when required by regulatory bodies
If you know your scope and the reason why you perform calibration, you can easily determine and assess the suitability of the reference standards to be used for those instruments that needed calibration. It also means that you are familiar with your reference standards.
For example:
ABC lab exists to provide a traceable calibration of measuring and test equipment under Dimensional, Pressure, and Electrical that can directly or indirectly affect the quality of our products.
The table below details the scope of capabilities and reference standards used.
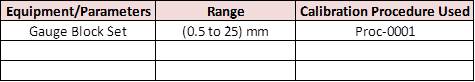
2. Recording System
I am sure before we start sending our instruments to an outside calibration lab, we already have a list of our measuring instruments.
If we have, this list is now our master list for our internal calibration laboratory (if you do not have one, you should create one, and take inventory of all the instruments for calibration).
It should include all the details of measuring instruments. This is now our starting point, we have now our inventory, we should determine where they are, who owns the instruments, and other details regarding the instruments like the calibration history file (instrument performance).
During this time, also take the time to put proper coding. This is now our monitoring tool. (in my past company, we have Calibration Management Software- the METCAL from Fluke which automates documentation and monitoring including the generation of calibration certificates). As a start-up, using an Excel sheet can already do the job.
Before, during, and after calibration, a recording is always a must. In calibration (whether internal calibration or external calibration), sometimes recording or documentation has more time to do than calibrating an instrument (so if you are a techy guy that hates writing or documenting a lot, this job will challenge you – just saying:-))
The Measurements Data Sheet (MDS) – Record Your Result!
You should have a form where your results and observations will be placed. I call this form a Measurement Data Sheet (MDS). Others call it a Measurement Data record, Calibration record, calibration worksheet, or company calibration form.
Why? Of course, to record and document your calibration execution. But another important reason is to have proper traceability regarding the performance, not just the execution but all the elements of the calibration system.
Just in case a problem arises regarding the instrument, we can easily trace back who perform the calibration and what procedure has been applied, and what data has been recorded etc.
MDS displays all the details regarding the UUC which includes: (Below is a sample Measurement Data Sheet (MDS))
-
-
- Instrument Name
- Instrument Description
- Brand/Type/Model/Serial #
- Equipment code
- The environmental condition- Temperature and humidity
- Date Received and Date Calibrated
- Location of the Instruments
- Range and Resolution
- The Standard used during calibration
- The Methods used in the calibration
- The owner or department who use the Instrument
- Results or data
- The person who performs the calibration
-
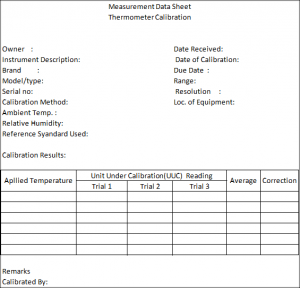
Note that applicable Document Control and Records Control applies to all calibration procedures and forms used and other documents that are part of the calibration system.
Once the data sheet is completed, you can convert it to a Calibration Certificate with almost the same but some added details and more presentable with the title Calibration Certificate (to be more formal and adequate, it should be aligned with the requirements based on ISO/IEC 17025:2017, General requirements for the competence of testing and calibration laboratories).
To view the ISO 17025 calibration certificate requirements and tips to properly review and interpret the requirements of a calibration certificate, visit this link.
To learn more about the Technical records used in calibration, visit this link >> Calibration Records
3. Calibration System (how we execute the internal calibration process)
The calibration system should detect and provide correction to any deficiencies related to calibration. A documented system that monitors and controls all measuring and test devices used in manufacturing a product, used in quality control or inspection operations to ensure conformance at given specifications or requirements.
Once we determine our scope, we will now specify what is the flow of our calibration process, how we can execute and control calibration jobs starting from collecting of the UUC to releasing of UUC with calibration certificates.
We will not just receive an instrument and then calibrate it immediately. We should have our first step before we proceed with calibration. Every step of a calibration process should be clear to us in order to have a smooth and documented execution.
In order to execute the internal laboratory calibration process properly and to have a clear view, below is a calibration process flow (or a plan) that I implement. You may change it depending on your needs but the steps or requirements should be maintained or more if necessary.
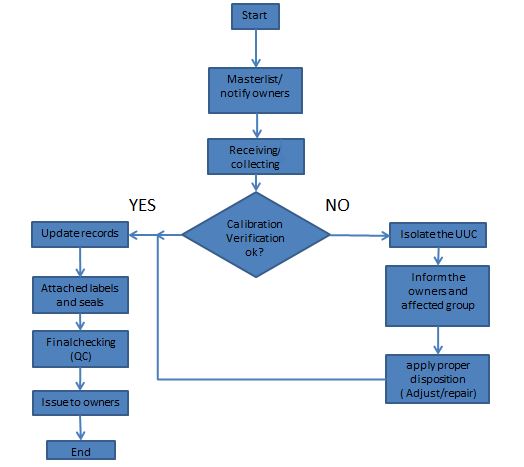
The calibration process presented above is the simplest, we did not consider putting other factors like what if we are not capable of calibrating a certain UUC, what if newly calibrated with an existing calibration certificate or totally damaged equipment?
These questions are usually tackled or solved by considering an additional process, but for simplicity, we will maintain it as is for the sake of this presentation.
4. Receiving of Measuring Instruments
Since we already have the master list, we can notify the owner and wait for them to submit the instruments (I assume that every UUC has its own user or owner). During this time, just make sure that all instruments that are due for calibration will be collected.
During receiving, make sure that all details are accounted for, below are the main details you need to acquire as a minimum (you need to have a specific form for this):
-
-
- List all the details about the UUC (name, date, make, model, serial no.)
- Check the status of the Unit Under Calibration (UUC), (this is how we will call here instruments for calibration). Power up the UUC and perform functionality check;
- Check for any defect and missing parts or accessories;
- Determine the requirements of the user, this is very important, you may encounter some ranges that you are not capable to perform during calibration where you need to know and inform to the owner.
- Determine and record the calibration interval;
- Place the calibration control tag or identification to avoid any confusion. The calibration tag includes the below details:
-
- your unique identification number
- location of calibration
- date received
- name of the owner
-
-
If the organization is small and only one or two persons is handling the instruments or UUC, this is no longer a problem to think but be sure to have complete monitoring of all the instruments under you.
Once the above details are all accounted for, let the user sign the form for acceptance and that they acknowledge that they are the responsible owner of the UUC.
5. Storage and Handling
Once you already receive the UUC, place them to a designated storage area, see to it that they are protected and with proper separation, label, or put a tag to ensure proper identification.
Proper identification and designation will ensure traceability in terms of monitoring the calibration status while inside the lab. Identification can be accomplished by placing a calibration tag.
Do not remove tag while it is still for calibration or inside the lab to ensure continuous monitoring of status. These statuses are:
-
-
-
Calibration status – is it calibrated or still ongoing calibration
-
For quality check
-
For Release
-
Who owns the instrument
-
Location of calibration – if under the parameter of Pressure, Mass, temperature, etc..
-
-
Make sure to observe proper handling when transferring.
After receiving and performing calibration of the UUC, check also your storage area, and see to it that UUC is protected from any environmental effects such as temperature, humidity, or electrostatic discharges.
During storage and transfer, one of the occurring environmental effects in terms of temperature and humidity is ‘condensation’ where there is a moisture build-up. Moisture can cause metals to rust, and rust can cause a short circuit. Learn more about the effects of temperature and humidity HERE.
Condensation happens when a cold instrument is suddenly brought in a warm environment. Therefore, make sure the storage area has also a controlled environment.
Observe also proper positioning or placing of UUC during staging (vertical or horizontal position) .

6. Internal Calibration Procedure (Methods)
This stage is where we determine all the requirements related to the actual calibration of UUC. After inspecting the received UUC, we will now determine how we can execute the calibration job.
Actual calibration can be performed or executed by following a documented calibration procedure. Calibration procedures is a controlled document. Whether it is an ISO 17025 procedure or an ISO 9001 procedure, it is based on:
-
-
- manufacturer manual or equipment manual,
- based on customer recommendations,
- based on experience with a similar type of equipment
- based on published standard documents or guides – this is a must to have or included as a reference, more so if you are planning to apply for accreditation
- or a combination of all.
-
After performing calibration, it is important to perform a verification. This is to check whether the results we obtain are within acceptable specifications. How to perform verification? read more in this link >> verification
If you will generate your own calibration procedure, ensure that it is properly validated, meaning you have determined it to be fit for its intended purpose, and of course, it should be properly documented. Check this link to learn more about validation.
Below are the contents that we should consider in generating a calibration procedure (as a minimum). These requirements are also applicable to ISO 9001 calibration procedure.
-
-
- Title of the procedure
- Scope
- References
- Required Instruments or reference standards (equipment used)
- Step-by-Step Procedure (which includes the Test parameters and tolerances)
- The uncertainty of measurements – this is one way to show traceability and confidence to our calibration results, also some auditors are requiring it.
-
Since it is a controlled document, we should not forget to include the author, revision number, a unique code for this procedure, the revision history, and more depending on the format in which your doc control requires you to add. Also, this generated procedure is required to be reviewed once a year or as necessary to ensure the use of an updated one.
Read more about the content of calibration procedure here >> calibration procedure
7. Labeling and Sealing
Once the UUC is calibrated, a calibration label or tag should be placed in the UUC as a sign of calibration. This will indicate the status of a calibrated Instrument. Most used labels are stickers that contain the below information (this depends on the laboratory):
Calibration labels should be clearly visible when attached. There are times when a UUC has a limited calibration or a calibration- not-required label, this type of label should be included.
But make sure that there are proper assessments and documentation. Also, regarding the UUC with has a smaller size where it is impractical to put the sticker, we can place it in its box or container.
It is also required to put a tamper-proof seal to protect the newly calibrated UUC from any unauthorized adjustments that can invalidate the calibration settings.
A calibration label printer is now available for faster and better printing of labels or stickers. More advanced calibration label printers are now available which is a fast and portable label printer that can be brought for site calibration.
8. Recall System (how can we get them back for recalibration)
Once a calibrated Instruments was returned back to the owner or released to be used again, you should have a recall system to get it back once it is due for calibration (this is applicable to a very large organization where there are different owners of instruments as per department or area).
This is one of the most difficult parts, where some owners do not care about their measuring equipment unless they are caught in an audit.
An effective recall system is one of the keys to having a successful calibration program where the owners of UUC are following the scheduled submission for recalibration without any delay (faithfully).
But usually, this is not always the case. I experience the same problem in the past where some UUC (Unit Under Calibration) owners always fail to submit the instruments on time.
To solve this problem, I implement the below system with the support of the quality department. This solution is to copy their bosses together with the quality team in the email and then directly address the bosses during follow-up.
This is what I call “the escalation method”. This is a scheduled email message where every email sent has a corresponding higher authority to be copied (CC).
Below are the steps:
1. The first notification is to the instrument owner of UUC only. This is a usual reminder to submit the instrument.
2. The second notification is a follow-up message, but this time, his supervisor is in ‘CC’ (copied).
3. 3rd notification is to message directly to the supervisor (with the instrument owner) and copy the quality manager (Quality Department).
Now, since the instrument is already overdue, and the quality team is notified, they will tag or confiscate the equipment and then perform corrective action to correct the system (not the person).
This practice is very effective based on the In-house calibration laboratory that I manage in the past.
9. Reference Standards (Equipment)
Reference Standards are the equipment we used to calibrate UUC, we compare and verify the accuracy of the UUC with respect to these reference standards. It has the capabilities of accuracy, stability, range, and resolution for the intended use.

A reference standard should have the below requirements:
-
-
- As much as possible at least 4 times more accurate than UUC to be calibrated.
-
- Must be calibrated by a laboratory accredited to ISO 17025 with the required Calibration Certificate
- Traceability to International Standards (BIPM)
- Properly maintained and verified (see element 13)
- Properly labeled, coded, and identified (related to element 7)
- A calibration program is established – calibration interval, PM schedules, history file
- Calibration records are maintained and updated – read more in my other post about calibration records >> technical records
-
Please check this link for traceability
10. Facilities and Environmental Control
There are no specific requirements regarding the layout of a laboratory, but there are requirements that we need to follow when choosing a lab, which is focused or concerned with the effect on the validity of results. This depends on the field or parameters that you will calibrate and the instruments/standards that come with it.
For example, if you are designing a lab that serves mass calibration, ensure that it is located on the ground floor to minimize the effect of vibration during calibration.
There are requirements that a lab must have as per ISO 17025: 2017, under clause 6.3 Facilities and environmental conditions.
Some requirements to consider that are related to the physical design of a lab are:
1. A room with a stable and clean environment.
2. There is enough space for proper separation or segregation in order to:
a. avoid confusion on what parameters to be calibrated during the staging of the instrument for calibration.
b. proper segregation or separation should be observed to avoid cross-contamination.
c. Some instruments or reference standards need to have a separate space because they are sensitive to temperature or humidity or other environmental factors. These can contribute to error in results and therefore needs to be separated. For example, temperature instruments cannot be mixed with dimensional instruments.
3. Controlled access to ensure security and confidentiality.
Environmental factors have a great impact on the calibration job. Whenever an environmental condition is not met, the calibration job should be stopped until it returns to normal.
Normal wherein, for example, a required temperature and humidity range that is prescribed is within the specified range. Since it is critical in our process, it is advisable to use a continuous recorder, a Thermo-hygrometer, to monitor and record.
Below are some examples of the environmental factors that we need to control:
-
-
- temperature
- humidity
- vibrations
- dust
- lightings
-
please check this link in my previous post to learn more about temperature and humidity.
11. Personnel Qualifications
All personnel (calibration technician or engineer) that performs test or calibration should have proper training, in short, should be competent enough where he can demonstrate the required calibration procedure when prompted.
They must have attended or oriented regarding calibration awareness as the first part of the training or exposure to calibration basics. Calibration awareness is one of the most important training that an organization should take regardless of the area you are in (like in production, planning, purchasing, and others). Of course, this is under quality surveillance.
Their competency is supported by their qualifications (experience, education, training, etc). Moreover, all of his training must be included in a record control file for proper monitoring.
To support Personnel Qualifications, we need the competence requirements such as:
-
- Authorizations
- Training records
- List of responsibilities and Job description
- Competency Level
Monitoring personnel competency (example: Training) is a continuous process and required to be updated or refreshed regularly.
To read more about this topic, visit my other post in this link >> Competence Requirements
Another important requirement under Personnel is to ensure that every personnel is observing Impartiality during the performance of calibration work, specifically those activities that have an effect or risk on the results of calibration done. A procedure that identifies this risk is called Risk to Impartiality procedure.
Impartiality means there is objectivity in the work performed where no pressure is involved. Also, the conflict of interest is eliminated or minimized. Read more in this link >> Risk to Impartiality
12. Data Retention (how long do we need to keep a record)
Data retention is important for every records control procedure, in calibration, data retention is very meaningful. Compare to other records, calibration records should be accessible easily even it is an old record, we need to collect and tabulate data specifically the data of reference standards for our history file.
This will provide a reference regarding the performance of a certain instrument, past data will help you to decide on how you adjust or provide a proper disposition specifically in determining the calibration interval of your instrument.
Usually, records retention is 5 years or longer.
13. Quality Control
What Quality Controls should be implemented to ensure the reliability of the reference standards used?
Reference standards are always used for measurement either inside the laboratory or outside the lab (on-site calibration). It is exposed to different environments and the handling of personnel.
Because of these factors, we must ensure that our reference standards are still in good condition outside and inside.
Below are the methods that we can implement for this:
- Intermediate Check – implemented every time the reference standard is sent out of the lab, during preventive maintenance or exposure to harsh environments.
- Preventive Maintenance – a scheduled maintenance
- Performing Measurement System Analysis
- Re-Calibration and verification
- Performing different methods of calibration and verification (replicate test)
Also, measurement results should be monitored and analyzed if the below situations are encountered:
- If there is an out of tolerance during calibration and verification or an out of tolerance is suspected.
- If a new instrument or standard has arrived from a third party calibration
- During a scheduled maintenance
- During the extension of the calibration interval
- If a new equipment is installed or transferred from different locations
Conclusion
Internal calibration, also known as In-house calibration is one of the most important processes within a company to provide a good product or services, including saving time and money, thus, implementation requires deep knowledge and dedication.
In this article, I have presented and explained the following:
- The Benefits of implementing an in-house calibration laboratory;
- Some challenges that you will encounter during implementation;
- Understanding and implementing the requirements of ISO 9001:2015 related to calibration
- The 13 elements that you need to have when implementing an In-house calibration laboratory.
Calibration involves so many factors to determine and consider when implementing such as:
-
- How to Handle out of tolerance calibration results?
- What to prepare during customer audits/internal audits?
- How to determine the calibration intervals?
- What will you do if you have a lot of overdue for calibration?
A smooth flow of operations is the result of a planned and documented calibration system, there should be a continuous improvement of every aspect to adapt to the ever-changing requirements and needs as per International Standards like ISO 9001:2015 and ISO 17025:2017.
What do you think?
Thank you for visiting my site, please leave a comment and subscribe.
Edwin

76 Responses
Serjy
Can you talk about BS50504 standard for calibration of welding machines.
edsponce
Yes, I will consider if i have the resources. do you have a copy of the BS 50504 standard?
Rob buxton
Hi,
Can we chat through email pls.
Trying to set up an in house calibration and could use your extensive knowledge to steer me in the correct directions
Regards
Rob
edsponce
Hi Rob,
This is my email address >> edwin@calibrationawareness.com
Thanks for visiting my site
Best regards,
Edwin
Alvin
Hi sir. Thanks for this post. We are planning to have an internal calibration laboratory in our company and with this post we can easily plan and justify our initiative by following your posts. Thanks a lot
edsponce
Hi Alvin,
You are welcome. I am very happy knowing that you are using this post as a guide. Hope that it can help you in more ways. I would be glad to know the outcome of your calibration journey in establishing your Internal Calibration Lab. Please do not hesitate to provide more feedback.
Best regards,
Edwin
Ich
Thanks you very much sir for the post.
edsponce
Hi Ich,
You are welcome. Thank you for reading my post. Please feel free to comment for any related concerns.
Best Regards,
Edwin
edmar
thank you sir..an apportunity to enhance and effective internal cal lab
edsponce
Hi Edmar,
You are welcome. Thank you for reading my post.
Best regards,
Edwin
Vinod Choudhary
Hi sir, very supportive post you provided each and every time.it will be helpful if you make a post on how to verify the performance of an equipment in between calibration, on the basis of that we can amend the calibration frequency on the basis of that verification results. Also if possible, please provide the structure for calibration certificates as per ISO/IEC 17025 for pressure gauges.
edsponce
Hi Vinod,
Thanks again for reading my post.
Regarding your concern, I have created a separate post where I explained how to amend the calibration frequency based on the calibration results. check out this link >> calibration interval
There is no specific structure for a calibration certificate especially pressure gauges that I am aware of, as long as you have the requirements of ISO 17025 regarding calibration reports and the requirements of the reference method you are using like DKD-R 6-1, you are ok with the structure of your calibration report.
I hope this helps,
Edwin
azwan
what is the suibtable size of lab for in house calibration?
edsponce
Hi Azwan,
Thank you for visiting my site.
There are no requirements for the size in any calibration lab. The size of a lab depends on:
1. The scope of your capability or the number of parameters that you can calibrate.
2. The number or size of your equipment or reference standards,
3. The budget that a lab can provide
4. The size of the company and the number of users or instruments it serves.
The things that you need to consider when looking for a laboratory room are:
1. Ensure that no cross-contamination is seen or experience.
2. Instruments should be protected and staged properly.
3. Some instruments or reference standards need to have a separate space because they are sensitive to temperature or humidity or other environmental factors. This can contribute to error in results and therefore needs to be separated. Example, temperature instruments cannot be mixed with the dimensional instruments.
4. You need also to consider that the lab must have a stable and clean environment.
5. There is no confusion on what parameters to be calibrated. There is enough space for proper separation or segregation.
Hope this helps,
Edwin
Vinod Choudhary
Thanks a lot sir for this useful information.
edsponce
Hi Mr. Vinod,
You are welcome.
Thank you for giving time reading my long article.
Best regards,
Edwin
John Getalla
Thanks Edwin, This a very interesting article to re-visit. I’ve been thinking about the sealing requirement “to put a tamper proof seal on the intrument to safeguard from adjustment which may invalidate results” ; any suggestion on how to do this in an industrial process environment wherin intrument/gauges were mostly subjected to low/high temp, dry/wet and CIP conditions.
edsponce
Hi John,
Thank you for reading my post.
I have seen a tamper-proof sticker that can withstand a harsh environment that you have specified but I do not know any suppliers. What we usually used like for example to some pressure transmitters, valves or gauges is the round lead seals with wires. The only way to access the instrument in this seal is to cut the wires. You can see this in this link>> Lead seal
Let me know if it suits your need.
Thanks and regards,
Edwin
Charlie
Sir. Good day. Because of your post I am considering to revise our in-house procedure. Thank you so much sir.
edsponce
Hi Charlie,
You are welcome. I am glad to know that my post have helped you in some way.
I appreciate your time reading my post. For any other related concern, please feel free to comment.
Thanks and regards,
Edwin
Charlie
Sir, we had audit findings from one of our customers on validation of our autoclave. Sir what is the difference of validation and verification and how do you perform validation of instrument. We already have valid plan but this will be the first time that i will be using it. Thanks.
edsponce
Hi Charlie,
Thanks for reading my post.
Verification is the confirmation that the final results of your measured value are within the specified tolerance or specifications.
For example, with your autoclave, you perform a verification with its temperature. As per your process tolerance, the reading should be within +/-5 Deg C. If your result is outside this tolerance limits, then it is FAIL and needs adjustment.
Validation is a more thorough process, it involves many processes. Validation is used to ensure that a given process, equipment, or a procedure is fit for its intended purpose.
Using again your autoclave as an example:
First, we will perform functional checks, physical check and the completeness of its accessories and parts.
Second, we will perform calibration of its pressure and temperature parameters, and afterward verification. Adjustment is done when needed.
Finally, you will need to perform an actual run or test for your product and see if it meets your specifications.
All these are documented and signed for approval and release by the authorized persons.
I have created a separate article about the difference of calibration, verification and validation in this link >>> calibration, verification, and validation
To supplement your procedure, you make check this guide from EURACHEM for method validation>> The Fitness for Purpose of Analytical Methods.
Best regards,
Edwin
Charlie
Thank you for the information sir edwin.
edsponce
Hi Charlie,
You are welcome. Happy to help.
Best regards,
Edwin
Simphiwe dladla
Thanks Mr
Powerful information
edsponce
Hi Simphiwe,
Thank you for reading my post. I am glad you liked it.
Best regards,
Edwin
Ariel
Hi Edwin,
Thanks for sharing this post and this information will help me to improve our internal calibration.
Thanks & Regards,
Ariel
edsponce
Hi Ariel,
You are welcome. Happy to know you have learned something in this article.
Appreciate your comment.
Thanks and regards,
Edwin
roniel s abiera
Hi Sir Edwin,
Can you share about measurement uncertainty in calibration? It would be a big help. BTW, your page is very helpful newbie like me.
edsponce
Hello Mr. Roniel,
I will include it in my future post. Is there a specific question about measurement uncertainty that you want to know better?
Thank you for visiting my site. I appreciate your comments.
Best regards,
Edwin
Rose Agana
Hi Sir Edwin,
Your site is very useful to me, since I’ was asked to manage a calibration lab. The lab would like to start with calibration of gas analyzers. Can you suggest a standard calibration procedure for gas analyzers?
Thanks and best regards,
rose
edsponce
Hi Ms. Rose,
Happy to know that my site is very useful to you. I appreciate it.
I am not an expert about gas calibration but I experienced performing calibration during my past work.
I am not yet aware of a standard reference guide, we use the manufacturer calibration procedure. Most gas analyzers have different setup designs where a dedicated accessory is used for calibration purposes only, therefore, it is advisable to follow the manufacturer calibration procedure.
I hope this helps.
Thanks and regards,
Edwin
joefer
Thank you so much sir.
edsponce
Hi Joefer,
You are welcome. Thank you for reading my post.
Thanks and regards,
Edwin
Taylor Wright
I like how you mentioned properly identifying a calibration system within a lab. My uncle now works in a lab and needs to work in a new calibration system and is worried it will take a long time. I’ll have to show him this so he can know what he’s doing.
edsponce
Hi Taylor,
Thank you for visiting my site and I very much appreciate sharing this article with your Uncle. I hope that my recommendations here will serve him well.
If there are any questions that you need to ask, please do not hesitate to comment further.
Thanks and regards,
Edwin
Joaquin
Hi, If I have a torque analizer in which manufacturer calibration certificate indicates units of measure in n.m (this is my standart) but I am using and verifying the torque in units lb.in (the standart can measure in different units) ; is this and issue due calibration certificate units are not the same that verification units? what would be the technical support to explain tha this is not and issue.
Thank you for your support.
edsponce
Hi Joaquin,
Converting units of measurement to one unit to another is not an issue. There are so many conversion factors that are used and accepted. Specifically from SI to non-SI units.
We are using non-SI units because of its practicality on its applications and ease of use for the user.
This is a normal process, as long as the conversion factor you are using is correct and acceptable. As you can see, even the instrument that you are using have different units on it where it is automatically calculated and converted.
As an explanation and support in case you face any issue, you can show these guides as a reference. This is from BIPM website >>SI Brochure
The only concern is that you should have a consistent rounding-off of decimals during conversion to avoid rounding errors. One tip is to perform rounding-off of answers only in your final result.
Another recommendation is, in order to remove this issue/doubt for you, is to have the instrument calibrated by your chosen lab to display the units that you are using (or inform them to include in the certificate the conversion factor of the units you need).
I hope this helps
Thanks for visiting my site.
Edwin
hytham alshaer
Hello,
Just I wanted to thank you a lot for this valuable and amazing in-house calibration post
edsponce
Hi Hytham,
You are welcome. I appreciate your comment. I am glad you liked it.
Thanks for visiting my site.
Edwin
PH Lim
Hi Edwin,
Thanks for your explaination for in house calibration.
For in house calibration, is it compulsory to calculate Measurement Uncertainty?
Do you provide training or consultation for set up a proper calibration lab internally?
Do you provide training for Measurement Uncertainty which is customerized based on the type of gages in a customer company?
edsponce
Hi PH Lim,
You are welcome. I appreciate your comment.
Regarding your question if it is compulsory to calculate measurement uncertainty for in house calibration, the answer is No, but with conditions.
The condition is based on the lab policy or procedure. Below are some reasons or conditions why we need measurement uncertainty:
1. We include it with the calibration results to determine a pass or failed decision. A more strict (but higher confidence) decision rule for compliance to specification.
2. To determine the quality of final results during calibration (very high or very low uncertainty means something is wrong in the calibration process)
3. If the ref standard used does not meet the TAR/TUR of 4:1
4. If required by another user in his process (to determine accuracy)
5. Required by auditors.
6. Ensure traceability
During my time when I was handling the In house calibration lab of a semi-con industry, as per procedure, we do not use measurement uncertainty as long as we maintain at least a 4:1 TUR for all calibration performed. But 1 time an auditor of TS 16949 (if I am not mistaken) still insist that we should include it in our calibration results.
The bottom line is, we need to calculate measurement uncertainty.
For the 2nd and 3rd question, Yes, I can provide the training/consultancy as per accepted conditions.
I hope this helps,
Edwin
Rose
Hi
1.Can you please show an example of a competency form and how to monitor competency through a form?
And what type of competencies should be on the form.
2. How do you address measurement uncertainty for a genomic lab that does genomic sequencing
edsponce
Hi Rose,
I do not have yet a competency form to be shared.
What I can share now is, what should be in a competency form (or a training form – you can name it as you like) and competency monitoring.
Competency form and competency monitoring form is dependent on our implementation of the personnel training procedure. It is possible that we do not need a competency form. It is based on the structure of our lab.
As per definition (Google), competency is the ability to do something successfully or efficiently.
Therefore, it is pertaining to “training” that we need to have in order to perform efficiently.
We need to have the training required for the job and monitor its effectiveness or results for every personnel involved.
We need to create a:
1. Training form – In creating a form, below are my suggestions that you need to include as a minimum:
a. Employee Name
b. Title/Position
c. Activity
d. Description/scope of training
e. Trainer and its signature
f. Duration of training
g. Remarks – either satisfied, not satisfied –need more training
h. Signature or approval of supervisor
2. Evaluation form – the purpose is to evaluate the result of the training that the personnel has completed.
Note: both could be in just a single form
This is what I can suggest regarding monitoring of competency (training report):
1. Every year (or as per your chosen frequency), a training plan and schedule will be conducted as per the recorded needs of the personnel involved.
2. Training will be conducted based on the training schedule and training plan for each personnel
3. A training evaluation form will be accomplished after the training, a record of an exam or actual demonstration for internal or a certificate from external training are included.
4. Based on the evaluation form, their level of skills will be assessed.
5. Level of Skills could start form Level 1- entry level to Level 4- subject matter expert, or marginal to superior (depends on how you grade each personnel) – the goal is to make all personnel to a ‘level 4’ or ‘superior’ level at a given period.
6. The results in the evaluation forms will be summarized and recorded in an excel sheet for proper recording and reporting.
An example of the type of competency that you can monitor and evaluate are:
a. Does the technician perform the calibrations as per procedure without supervision
b. Does the technician evaluate and calculate measurement uncertainty effectively
c. Can the technician generate calibration procedures
d. Can the technician perform preventive maintenance as per the procedure?
Please remember that this is only a suggestion that is effective as per my implementation. The details or process could be more or less based on your structure and needs.
For Question # 2, I cannot comment more on it because it is not in my scope of experience. What I can suggest is that if you are familiar with the GUM 1995 or JCGM 100:2008 , the process of evaluation is the same. You just need to know and understand the sources of error.
I hope this helps.
Thanks for visiting my site.
Edwin
Rose
Super helpful and much appreciated
edsponce
Hi Rose,
Happy to help.
Best regards,
Edwin
aliya
Hi,
recall system will be notified equipment owner via email 3 times. (10days before due, on the due, and a day after due) but still the equipment failed to submit to lab to calibrate.
at the end the recall system will be showing a lot of overdue and it seems the system is failed.
what will you suggest to counter the overdue equipment for calibration?
how to explain to auditor it is not cal personnel fault?
edsponce
Hi Aliya,
Recall system is one of the key to have a successful calibration program if the owners are following the scheduled submission of instruments for recalibration without any delay (faithfully). But usually, this is not always the case. I experience same problem in the past where some instrument owner always fails to submit the instruments on time.
To solve this problem, I implement below system with the support of the quality department. This is what I call “the escalation method”. This is a scheduled email message where every email sent has a corresponding higher authority to be copied (CC).
Below are the steps:
1. The first notification is to the instrument owner only. This is a usual reminder to submit the instrument.
2. Second notification is a follow-up message, but this time, his supervisor is in ‘CC’ (copied).
3. 3rd notification is to message directly the supervisor (with the instrument owner) and copy quality manager (Quality Department).
Now, since the instrument is already overdue, the quality team will tag or confiscate the equipment then perform corrective action to correct the system (not the person). The corrective action is what you can show to the auditor.
You should demonstrate how to ensure that overdue equipment is not being used in the production or in the line.
That is my suggested system if you want to apply. This is very effective for me, in the setup of the company I worked for in the past.
I hope this helps,
Edwin
Abdullah
Thank you Mr. Edwin for the valuable information. Regarding this implementations, is it applicable to the solar system instruments?
edsponce
Hi Mr. Abdullah,
You are welcome. Thanks for reading my article.
I am not familiar with the instruments you are referring to, but if it is a measuring instrument that needs calibration, then yes, it requires calibration program to properly manage the calibration implementation that I discuss here.
If you are not sure whether the instruments you refer needs calibration or not, you can visit my post in the link below where I explained what instruments need calibration and what instruments that do not need calibration. Check it out here >> https://calibrationawareness.com/calibration-not-required-implementation-guide-for-in-house-calibration
I hope this helps,
Edwin
Sherlock
Hi Sir,
Thanks for such great articles and very structured information!
I would like to ask if it is needed to “calibrate” go-or-no-go gauge/fixture? what is the correct method to verify this gauge?
Thanks!
edsponce
Hi Sherlock,
You are welcome.
A Go-no-go gauge is used to determine if a product is acceptable or not, we based our decision here therefor it is needed to be calibrated.
There is so many kind of go-no-go gauge, as long as you use a calibrated instrument with good traceability, where you can compare the measurement result, it is already a good way to use it as a verification standard.
Example is a block of metal (gauge block) used as a go-go-go fixture measured and verified using a calibrated micrometer.
I hope this helps,
Edwin
Mary
Hello Edwin,
Congratulations for your article, its great and a lot of good information. I wanted to ask if this procedure could be applied to perform the routine checks of scales as well. In this case how would I evaluate the data, to see if it passes or fails a test. I’ve read I should use tolerance limits or control limits but based on what will I be able to determine that? Thank you in advance.
edsponce
Hi,
Thank you.
Can you tell me what procedure you are referring to?
Evaluation of data if it is passed or failed is based on your acceptance criteria or tolerance limits. Once you have determined your tolerance limit, the next thing is to compare the measured value if it is within the tolerance limits.
When we are performing calibration of scales, we usually used the tolerance limit provided by the user so this is not a problem.
As per your concern, I believe you want to know how to determine the tolerance limit of your scale.
You can do it in 3 different ways:
1. Through user or process tolerance requirements.
2. Check the user manual of the scale for its specifications. Check the accuracy part. But most manufacturer does not indicate directly the tolerances, therefore, you need to calculate using the guides in No.3 below.
3. Check the Accuracy Class of the scale determine what Class the scale belongs to (Class I, II, III, or IIII),. This is usually given also in the manual under specifications. Check OIML R 76-1 for the MPE (maximum permissible error) as per accuracy class.
Actually, you need to learn how to calculate the MPE because the acceptance criteria are based on many factors like accuracy class designation, max. range and resolution. This is presented in the OIML R76 guide.
Check out my other post where I presented how to calculate the tolerance limit of a balance if it is not given in its specifications >> balance verification
I hope this helps.
Edwin
Luis Cardenas
Hola Edwin,
Muy interesante y profesional tu información, gracais.
Quería preguntar si tienes información mas especifica sobre la evaluación continua del desempeño del personal y sus competencias.
Saludos,
Translation from google: I wanted to ask if you have more specific information about the continuous evaluation of the performance of the staff and their competencies.
edsponce
Hi Luis,
I have translated your message and below are my answer as per my understanding..
Below are my inputs about competency and its monitoring.
The requirement is to have competent personnel. And you need to prove that they are competent.
Competency Monitoring- but how to monitor? Prepare all below records.
a. Yearly training
b. Actual observation during internal audits
c. By determining the level of competency – as an example, a fully competent person has a level 3 rating. Providing a “level” is how you measure their competency.
i. “Your goal is to make all personnel to a level 3 rating”. Create criteria, if they can do all the criteria then they are level 3. see example of level below:
level 3 -expert
level 2- needs supervision
level 1 -trainee
If they are not yet capable of some of the criteria, then they will fall to a lower level depending on your assessment.
Example of criteria for level 3 are: (you can add more based on what you feel is needed- these criteria will tell if they are level 3 or lower)
1. Can perform without supervision
2. Can generate calibration procedure
3. Can calculate measurement uncertainty
4. Can analyze and troubleshoot problems
5. Can train others
If all your personnel are level 3, then its done, all you need to do is to maintain it and keep the records.
I hope this helps,
Edwin
Vivek Ajjarapu
How does one estimate the cost of starting an internal calibration lab for test and measurement / fab type equipment? Cost of standards, recurring costs etc.
edsponce
Hi Vivek,
There are so many factors involved but I will share the most simple. Estimation of the cost will start based on how many instruments are needed to calibrate. Once you have listed the price of calibration for each instrument (UUT), then look for a calibrator that can calibrate each UUT.
If the total price of calibration service is almost the same when purchasing a calibrator at a given period, then it is a good decision to purchase the standard. Some calibrators are expensive where it needs 2 to 3 years before you can cover the whole costs. But in the long run, you will see the benefits. See my sample justifications in this link >> https://calibrationawareness.com/11-ways-to-use-fluke-754-fluke-754-review-of-capabilities
Other costs that you need to consider which are direct only to calibration are:
1 cost for personnel- training and manpower
2. recalibration of calibrators (depends on frequency) -this is one of the recurring costs
3. repairs and upgrades
4. computer and office supplies for certificates
5. logistics
I hope this helps
Edwin
Nathan Marckini
Thank you for putting this information together, it has been very helpful. I am currently in the process of building an in house calibration program. Are ISO17025 and ISO9001 the only governing documents that I should be following? Also is there any accreditation or licensing that I would need to look into?
edsponce
Hi Nathan,
You are welcome.
There are other governing documents that you can follow, like Z540-3 and other standards that depends on the industry you are in. For example TS16949 for automotive industry and ISO 13485 for medical devices.
But for more in-depth implementation that mainly focus in calibration management is ISO 17025 and Z540-3. ISO 17025 is the internationally recognize Standards used by accreditation bodies.
You may approach the accreditation body near you if you plan for accreditation to have more guidance.
Good luck for building your in-house calibration program.
Thanks for reading my posts.
Best Regards,
Edwin
Rashida
Hi Edwin,
Your article deserves to be applauded, it is so comprehensive. I am working with a company which sells laboratory and medical equipment. In view of value addition to our customers, we want to provide calibration services over and above our verification and maintenance programme. We want to submit an accredited calibration certificate. As an initial project we want to offer mass and temperature calibration services for balances, ovens, autoclaves, incubators among others with a minimum investment.
Do have any paper on such topic or can you advise on the best approach for implementation of such project.
Thanks and best regards
edsponce
Hi Rashida,
Happy New Year! Thank you for your comments, it motivates me more to create and share more helpful content. Glad to know that you are now planning to become an accredited lab.
In order to provide an accredited calibration certificate, then your goal is to become an accredited laboratory. If you already have the reference standards for the mentioned scope, then the next move is to implement the requirements of ISO 17025:2017.
The best approach for me is to conduct first a gap analysis in order to determine what you already have and what still needs to be done. You will use the ISO 17025 requirements as a checklist in performing gap analysis. I have created a summary of documents and procedure list in this link>> ISO/IEC 17025:2017 Requirements: List of Documents Outline and Summary
I do not have yet a complete guide for implementation but I can help you along the way. I already started most of the implementations here in my website.
I hope this helps,
Edwin
Randy Reyes
Hi Sir Thank you for the useful information.
Regarding in calibration documents and records, can we consider softcopy even without sign of person who calibrated and approved signatory?
will propose the electronic documents in the future in our company.
edsponce
Hi Randy,
You are welcome. Thanks for reading my posts.
Yes we can. We are now transitioning to a paperless implementation of documents so it is a good option to propose.
But it depends on how you can implement the control of records and documents.
You need to consider the following control in addition to the requirements of “Document control procedure” you already have:
1. The database where you save the soft copy is secured.
2. There is a system of approval and monitoring that is implemented before you use the document.
3. Only a read copy is shared, cannot be edited by an unauthorized person.
4. A database back-up and maintenance is regularly performed.
With regards to soft copy for calibration technical records, make sure that the personnel who performed the calibration is seen in the record. I also suggest to include a digital signature (or some type of approval that you can implement) before release. Approval of documents or records specifically for technical records should be evident.
I don’t experience it yet, but there are software or a Calibration Management System Tool that you can use to do this type of work.
I hope this helps,
Edwin
Sergey
Hi Edwin.
Please explain how to draw up the Uncertainty budget.
Can you give an example? I can provide a calibration certificate for the 8508A multimeter
edsponce
Hi Sergey,
I believe you understand how to calculate measurement uncertainty.
Below are the components or contributors for multimeter that you can include in your uncertainty budget:
1.Repeatability – standard deviation from repeated measurements
2.Resolution of the UUT
3.Resolution of the standard
4.accuracy specification of the standard(from manufacturer specifications)
5.reference standard uncertainty- expanded uncertainty of the standard from calibration certificate
6.stability (if available from the manufacturer specs)
You can add more depending on your assessment of other contributors.
I hope this helps.
Edwin
Syed Waseeh
For master equipment calibration…4th step calibration allowed or not?? International Standard, National Standard, Local Standard,Local Standard and then our master equipment??
MHAY
This is very informative, Sir! We are currently trying to expand our scope (from flow meter calibrations) by adding pressure and mass calibration. Using your blogs to guide us through some studies.
edsponce
Hi Mhay,
You are welcome. Thanks for reading my articles. If there are any doubts you have, you can comment further.
Thanks and regards,
Edwin
Biruk Girma Hailemariam
Dear Sir
i would like to appreciate your knowledge of calibration and you are a real calibration scientist and could you share/ tell us your expertise area as well any challenges or difficulties that you face during ,on process and after implementation of ISO:ICE 17025/2017 new version ?. right now i am a new scientist for radiation science but i was good expertise in temperature calibration so that please share me your way to accreditation on radiation calibration or any document or contact person on the field of radiation science
with regards
Biruk Girma Hailemariam .
edsponce
Hi Biruk,
Congrats on your new position. I will try to share in my future posts regarding the challenges of implementating of ISO 17025:2017.
Regarding documents related to radiation, sorry to tell but I do not have any references. But speaking of accreditation, the part where you will see a big difference would be the calibration related procedures, mostly methods and the related requirements for each instruments. Technical and quality requirements still the same.
Thanks for visiting my site.
Thanks and regards,
Edwin
Amy Saunders
Very well, then. It feels really amazing to realize that in-house calibration can actually enrich the level of security for our precious equipment too. The main flowmeter at my cousin’s kitchen hasn’t been working properly lately and he wonders what can be done about it. As for me, calling a service provider could be one of the things he can do to resolve the issue.
Scott
Are there any requirements for software used as a management system?
edsponce
Hi Scott,
There are no requirements in terms of specifications but there are requirements in relation to quality. The software must be controlled. It means it should be monitored and approved for use. It is controlled under control of documents.
I hope this helps,
Edwin
William Frankum
Hi Edwin,
Your site is brilliant thank you very much.
I have been having issues with calibration date labels falling off when items are used in some oily environments, boxes go missing and have resorted to etching the database number on them for identification. This can then be used to look up the item to see when it was last calibrated. My question is: is this enough or must the items have the due date on them?
Thank you
Will
edsponce
Hi William,
There are no exact rules for labeling. As long as the calibration status of the unit is identified and protected from being tampered, then yes, it is enough. You just need to update or align your procedure with it so that it is clear to everyone (specially auditors) that it is how you identified UUT calibration status. I encountered the same on a pressure gauge, what we do is to engrave the calibration date and due date on a metal plate then use a metal string to attached it.
Sorry for the late response, and thank you for visiting my site.
I hope this helps.
Edwin